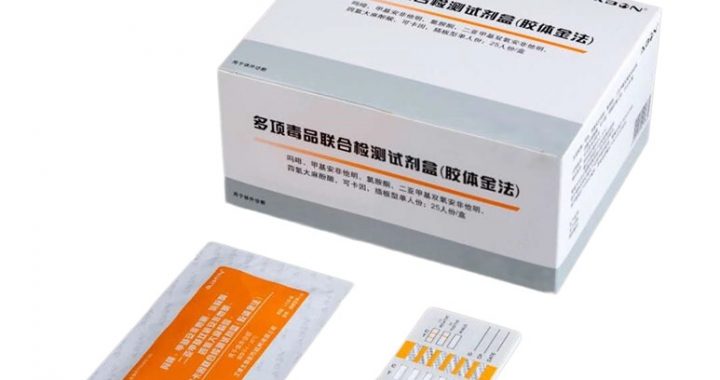
On-site narcotic drug inspection kits rely on specialized rapid screening methods to deliver quick, preliminary identification of controlled substances—critical for law enforcement, border security, and emergency response. These methods prioritize speed, portability, and ease of use, enabling personnel to make immediate decisions in field settings without relying on laboratory analysis. Unlike confirmatory techniques (e.g., GC/MS), rapid screening focuses on qualitative detection, flagging the presence of target narcotics (e.g., heroin, morphine, fentanyl, oxycodone) to guide further action, such as seizure, detainment, or referral for lab confirmation.
Core Rapid Screening Methods
The effectiveness of on-site kits hinges on four primary screening methods, each tailored to different sample types and operational needs:
-
Immunoassay Strip Testing: The most common method, using antigen-antibody binding to detect narcotic metabolites or parent compounds. Test strips are dipped into liquid samples (urine, oral fluid) or moistened with dissolved powder/residue. A colored line appears if the target narcotic is present, with results in 2–5 minutes. It excels at screening for opioids, cocaine, and amphetamines but may have cross-reactivity with certain medications.
-
Colorimetric Chemical Reactions: Involves adding reagent solutions to samples, triggering a visible color change specific to the narcotic. For example, Marquis reagent turns purple in the presence of heroin or morphine, while Scott reagent turns blue for cocaine. This method works for powders, crystals, and residues, with results in 1–3 minutes. It is cost-effective but requires careful reagent handling due to toxicity.
-
Handheld Raman Spectroscopy: A non-destructive method that uses laser light to analyze molecular vibrations of substances. The device compares the sample’s spectral fingerprint to a built-in narcotic database, displaying results on a screen in 10–30 seconds. It works for solids, liquids, and packaging residues, offering high specificity for fentanyl and synthetic opioids. However, it is more expensive than strip or colorimetric tests.
-
Ion Mobility Spectrometry (IMS): Detects ions produced by vaporized or aerosolized narcotics, separating them by mobility in an electric field. Portable IMS devices can screen air, surfaces, or small samples in 5–15 seconds, making them ideal for luggage or vehicle searches. They are sensitive to low concentrations of narcotics but may be affected by environmental humidity.
Key Features of Screening Methods
-
Ultra-Fast Turnaround: All methods deliver results in 30 seconds to 5 minutes, enabling real-time decision-making in field operations.
-
Minimal Sample Preparation: Requires little to no pre-processing—samples can be tested directly (powders, strips) or with simple dilution (liquids).
-
Field-Ready Design: Methods are integrated into portable kits (weight 0.5–5kg) with no need for external power (battery-operated or reagent-based).
-
Balanced Specificity: While less precise than lab methods, they offer sufficient specificity to reduce false positives, with cross-reactivity profiles documented in kits.
Scope & Applications
-
Law Enforcement Patrols: Roadside screening of suspected drug users, vehicle searches, and raid responses to identify narcotics quickly.
-
Border & Customs Inspections: Screening of cargo, luggage, and parcels for concealed narcotics, especially fentanyl and heroin shipments.
-
Emergency Response: HAZMAT teams and first responders using IMS/Raman methods to detect narcotic vapors in hazardous incidents.
-
Correctional Facilities: Screening visitors, mail, and cells for contraband narcotics using strip or colorimetric tests.
Best Practices for Use
To ensure accuracy, users should: 1) Follow kit instructions for sample volume and reaction time; 2) Use fresh reagents/strips (check expiry dates); 3) Confirm positive results with lab testing when legal action is required; 4) Store kits in cool, dry conditions to preserve reagent stability.